January 2023 – Huaxiang Group, a leading 3D Printing solution provider with expertise in medical industry, had received the category 3 medical device clearance from NMPA (National Medical Products Administration) for its Tantalum fusion cage additively manufactured on Farsoon metal machine. It is the very first NMPA approved Tantalum orthopedic implants built by metal powderbed fusion technology in China.

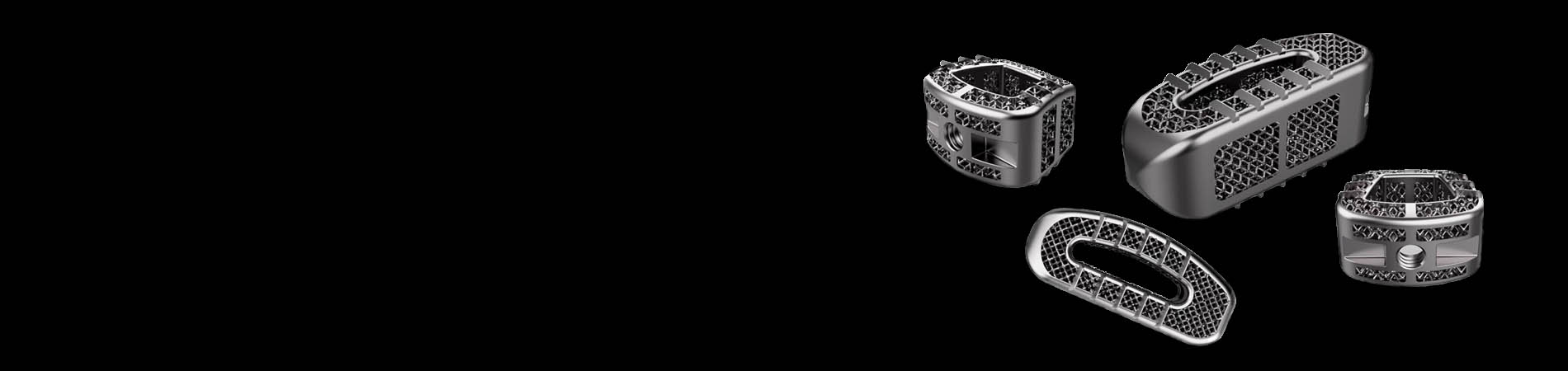
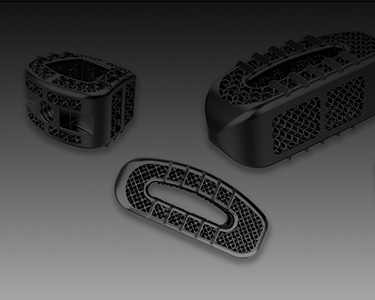
(Figure 1) Tantalum interspinal fusion cage with porous structures designed for 3D printing (image courtesy Huaxiang Group)
Known as a specialized metal material with excellent biological inertness and compatibility, stable chemical properties and abrasion resistance, the tantalum is an ideal metal material for medical implants. Since its first use in orthopedics in 1940s, the tantalum has been used in many kinds of medical devices for nearly 80 years.
Due to the extremely high melting point (over 3,000°C), high density (16.6 g/cm3) and elastic modulus (185.7 GPa), the pure tantalum material is quite challenging to process for use in the medical field. In the past 50 years, the conventional metal tantalum parts were manufactured through a complicated process: powder metallurgy or electron beam melting, deformation, welding and heat treatment.
With the application development of tantalum orthopedic products such as femoral head repair, cranial implant and joint prosthetics, the market has kept pushing the new designs and advanced manufacturing. The tantalum implant with porous structure is proved in clinical practices to reduce stress while providing sufficient mechanical strength; it also encourages bone and vascular tissue growing into the porous structure. The chemical vapor deposition process was then developed for producing commercialized porous tantalum implants for medical use; however, with the limitation of the technology it is only able to deliver standard end products, with a high manufacturing cost.
Compared to the previous manufacturing processes, Farsoon 3D printed tantalum porous interspinal fusion cage solutions developed by Huaxiang Group showcases many unique advantages from design to manufacturing:
• The additively manufactured implants can be fully customized and produced according to patients’ conditions. The trabecular micro structure can achieve a high porosity of 68-78% to promote bone tissue and vessel fusion.
• The elastic modulus of the 3D printed tantalum implant is highly comparable to human cancellus and trabecular bone. It offers excellent stability, bio-mechanical compatibility as well as reduced stress-shielding.
• Precisely produced using the digital model, the implants can achieve high size accuracy, internal-structure, and designated roughness which only require minimal post processing.
• Excellent load-bearing capability. The additively manufactured implant is ready for immediate load-bearing; with high toughness, good plasticity and fatigue resistance.
• Sustainable manufacturing with high material utilization.
• Improved efficiency with optimized manufacturing workflow.
• Reduced part lead time and cost.
With years of technology innovation and clinic practices in the medical field, Farsoon and Huaxiang Group has made a list of achievements in additive manufacturing application:
• World’s first knee replacement surgery using 3D printed tantalum implant in 2017;
• World’s first 3D printed implant surgery using degradable Zinc alloy material in 2020;
• First NMPA approved porous titanium spinal fusion cage built by powderbed fusion technology in 2021;
• NMPA approved porous titanium interspinal fusion cage in 2022;
• Over 14,000 customized surgical guide cases by end of Jan 2023.
With the continuous innovation in technology and materials, Farsoon experts are ready to provide all the support you need to accelerate projects in healthcare industry. During May 2-4th, 2023, Farsoon will be showcasing this tantalum porous interspinal fusion cage product at Booth 2224 at Rapid+TCT in Chicago, register now to get your free ticket. Inquiries and customers interested are welcome to contact globalinfo@farsoon.com for more information.
About Huaxiang Group:
Huaxiang Group is the industrial leader of medical 3D printing application in China. With an established medical industrial chain including materials research and development, software, additive manufacturing, post processing, etc., Huaxiang Group is ready to offer hospitals and doctors with comprehensive, accurate and efficient industrial grade medical 3D printing solutions.
About Farsoon:
OPEN FOR INDUSTRY – Farsoon Technologies, founded in 2009, is a global manufacturer and supplier of industrial level polymer and metal laser sintering systems. Farsoon is the leading supplier of industrial AM technology in China with increasing growth in the international market. In 2017, Farsoon released the first of a new series of machines under the revolutionary Continuous Additive Manufacturing Solution (CAMS), and established Farsoon Technologies-Americas in Austin, Texas, USA. In 2018, Farsoon Europe GmbH was established in Stuttgart, Germany to expand direct operations to Europe. Farsoon is committed to developing AM towards its true manufacturing potential and providing customers with best-in-class systems, materials, software, applications and services. Learn more: www.farsoon-gl.com