In the medical industry, additive manufacturing has enabled fully digital, customizable, precise “design to manufacturing” solutions compare to conventional production process, especially in the cases of creating sophisticated parts or internal structures to match each patient’s specific anatomy.
Yet, manufacturers of medical 3D printing must meet intense regulations from the medical communities. From the beginning, manufacturers must assure that their products or devices are made from materials that comply with biocompatibility requirements for each application.
From customized surgical models to Orthosis device to medical tools to personal protective equipment (PPE), Farsoon plastic laser sintering has assisted the medical industry with production parts made with Farsoon’s flagship polyamide material powder FS3300PA. This PA12 based material features excellent mechanical properties and bio-compatibility. FS3300PA has received medical grade evaluation to be suitable for intracutaneous reactivity, irritation and skin sensitization, FS3300PA is identified with good chemical stability and durability for production of medical grade devices. The as-printed parts can be further post-processed with blasting, ultrasonic cleaning, UV light disinfected, high heat and pressure as needed.
Using FS3300PA material and Farsoon 403P system, the medical division of Huaxiang Group has established a solid background in medical 3D Printing. Its surgical tools and components production has surpassed 4000 parts per year. Huaxiang Group has successfully supported a wide variety of innovative medical projects in research & development, surgical planning and operational tools for short-term skin or mucosal membrane contact.
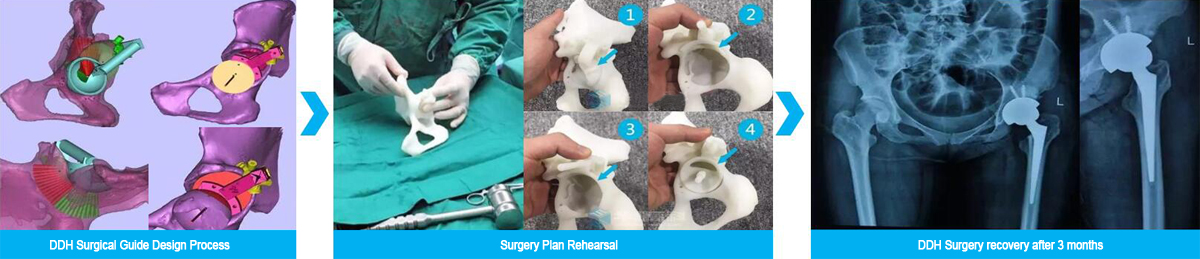
Figure 1: 3D Printed surgical guide for DDH Surgery (Developmental dysplasia of the hip). Image courtesy Huaxiang Group
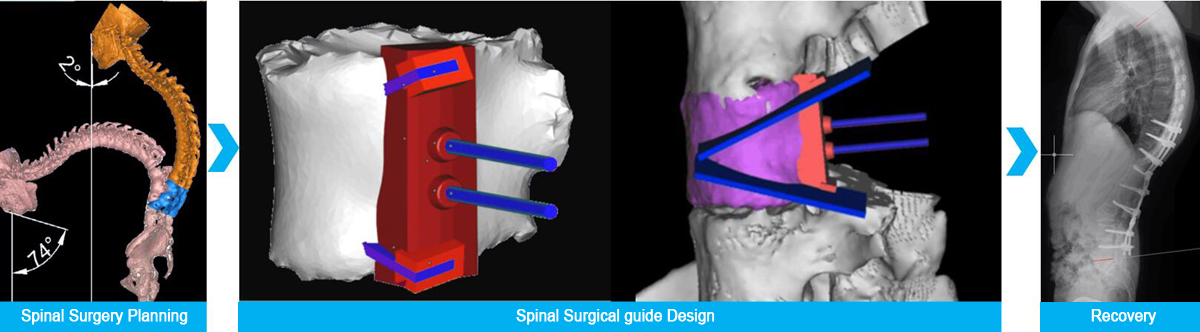
Figure 2: 3D Printed surgical guide for spinal surgery, image courtesy Huaxiang Group
Since the first introduction of Flight Technology laser sintering innovation during TCT Asia 2019, Farsoon has also continued the bio-compatible material development with fiber laser to develop highly productive solutions with improved detail resolutions for medical markets. Benefiting from Farsoon’s know-how in polymer AM material and partnering with medical industry partners, Farsoon’s Flight Technology also offers customer medical grade (ISO 10993) bio-compatible PA12-based polymer material solution for advanced medical applications.
During beta-testing with Flight 403P production systems, service bureau customers have intensively operated and investigated the material, which shows a balanced performance of strength, durability, tested medical-grade bio-compatibility for irritation and skin sensitization for medical device fabrication. Now entering the commercial operation phase, Flight Technology has proved to be a highly-productive manufacturing tool, even cost competitive with injection molding when it comes to low volume production.
Farsoon strives to offer industrial partners with bio-compatible, highly productive material-machine solutions for functional applications the medical field. Inquiries and interested customers are welcome to contact globalinfo@farsoon.com for more information!